
This site
is mobile
responsive

In envisioning Malaysia as a global business, innovation, and talent hub for advanced manufacturing, the biomedical cluster emerges as a key contributor to this ambitious vision. The focus is on transitioning into higher value manufacturing, where the competitive edge lies in know-how and skills rather than just cost. A pivotal aspect of this strategy is the development of the biopharmaceutical sector, particularly in the rapidly evolving landscape of biosimilars and biologics.
The world has witnessed extraordinary medical advancements over the past two decades, with breakthroughs like gene therapy, stem cell treatment, cancer immunotherapy, and innovations in 3D printing shaping the future of healthcare. In this context, the term ‘biopharmaceuticals’ takes centre stage, encompassing therapeutic products derived from living organisms. The global market for biosimilars and biologics is on a robust growth trajectory, with Malaysia poised to play a significant role.
While Malaysia currently hosts only one (1) foreign company producing biosimilar products, the biosimilar market in the Asia Pacific region is projected to soar from USD1.6 billion in 2023 to USD6.54 billion by 2028.
Concurrently, the Asia-Pacific biologics market is expected to reach USD141.3 billion by 2030. However, entering the biologics sector poses challenges for Malaysia, necessitating substantial long-term investment and technical expertise. Leading nations such as the USA, Germany, the UK, China, India, and Japan spearhead biologics manufacturing, spurred by continuous research and development and innovation, technological advancements, and a surge in chronic diseases like diabetes, cancer, and heart disease.
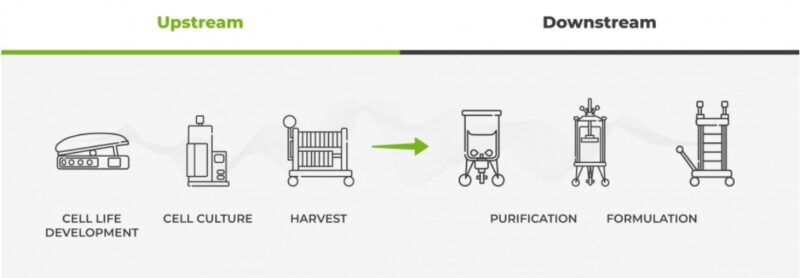
Biologics, unlike generic drugs synthesised chemically, undergo bioprocessing—a biological manufacturing method utilising biological resources such as living cells or microbes. The biologics production journey comprises upstream and downstream processes. Bioreactors and fermenters play a pivotal role in the upstream process, establishing a sterile environment to sustain and multiply cells, producing the desired protein product. Commonly, bacteria, yeast, mammalian cells, and insect cells are employed, with mammalian cells, particularly hamster cells, preferred for their compatibility with human proteins. The cell culturing process involves multiplying cells within the bioreactor until optimal levels are reached, marking the conclusion of the upstream process in biologics/biosimilars. Cultured cell capacities in bioreactors can reach millions or billions. Subsequently, the downstream process purifies relevant proteins from the cell mass, meeting stringent purity and quality requirements before formulating therapeutic products (refer to Diagram 1).
SUT are emerging as a preferred alternative in biologics production, gaining significant traction in advanced economies like North America, Europe, and leading Asia-Pacific nations such as China, India, and Japan. This shift is driven by the biologics industry’s progress in offering targeted and precision medicine, exemplified by advancements like cell and gene therapy, intensified by increased research and development activities.
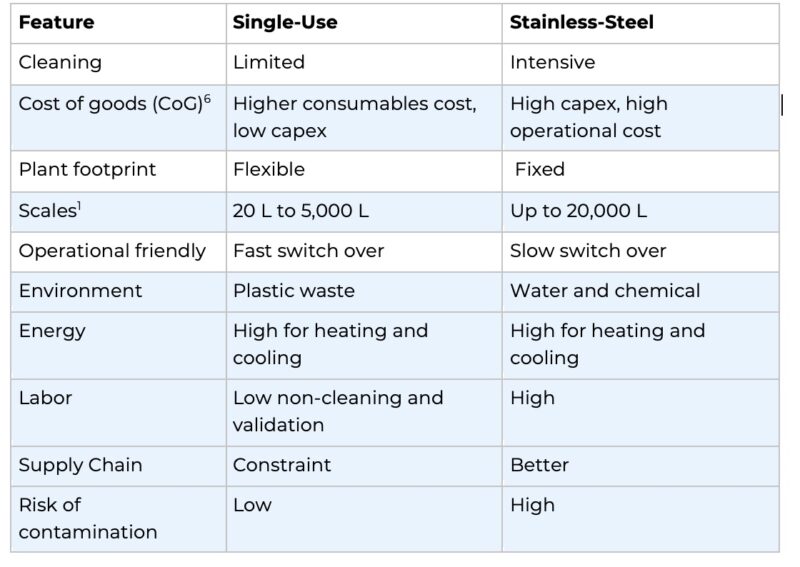
Conventionally, large pharmaceutical companies, operating with economies of scale, utilised large stainless-steel bioreactors (SSB) equipped with centrifugation system up to 20,000 litres in capacities. However, this approach of separation proved economically unviable for volumes below 5,000 litres; limiting the participation of small to medium-sized companies in biologics/biosimilars due to their challenge of achieving economies of scale with their smaller capacities.
The single-use technology (SUT) offers a more straightforward, quicker, and cost-effective path to achieving production capacity when compared to traditional stainless-steel equipment. The advantages of SUT extend to both capital investment and operational expenses. Conversely, stainless steel bioreactors are typically tailored to a specific product line. For facilities requiring adaptability, SUT proves advantageous, as it allows for easier adjustments based on alterations in process conditions or variations in drug types. The decision between SUT and stainless-steel bioreactors is contingent on the drug’s overall development, market dynamics, and considerations for batch size. Several key factors that comes into consideration for the optimal technology for biopharmaceutical production includes:
The late 1990s witnessed the transformative introduction of SUT, enhancing the efficiency of biologics/biosimilars production at smaller capacities. SUT is designed to reduce capital investments and boost flexibility for swift clinical operations compared to conventional stainless-steel bioreactor (SSB) technology. Economic evaluations, prioritising speed-to-market, outweighed SUT over the costly stainless-steel facilities. This led to the apparent adoption of single-use systems for clinical manufacturing.
Recent years witnessed the prevalence of single-use and stainless-steel equipment in flexible biopharmaceutical manufacturing, driving the establishment of hybrid facilities. Hybrid facilities commonly deploy stainless steel bioreactors for upstream processing and single-use skids for downstream processing, enhancing separation and purification. In the future, biologics manufacturers will embrace flexible, hybrid facilities, accommodating both SUT and SSB technologies. This strategy optimises efficiency, flexibility, and cost-effectiveness in biologics production.
While the biologics sector presents a substantial barrier to entry for developing countries like Malaysia, a hybrid bioprocessing approach, combining SUT and stainless steel, proves advantageous. Success in this venture relies on strong support, including robust R&D infrastructure, available and attractive funding schemes, a STEM talent pool, and strategic partnerships. Malaysian companies in biologics production can chart a successful path by adopting hybrid facilities, suited for handling various drugs and aligning with the country’s industry goals.
For more information, please get in touch with the MIDA Life Sciences and Medical Technology Division at https://www.mida.gov.my/staffdirectory/life-sciences-medical-technology-division/
