This site
is mobile
responsive

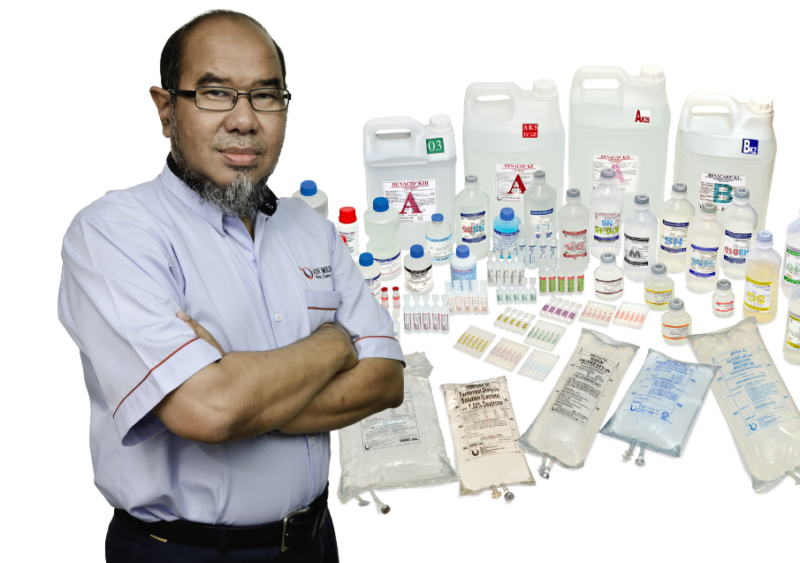
A local manufacturer of pharmaceutical products in Malaysia, Ain Medicare Sdn. Bhd. (AIN MEDICARE) is synonymous with producing sterile and non-sterile products, ranging from Intravenous Infusions, Antimicrobial Infusions, Small Volume Injections, Irrigation Solutions, Haemodialysis Concentrates, and Peritoneal Dialysis Solutions.
Established in 1994 as Malaysia’s sole manufacturer, AIN MEDICARE began with only 80 personnel, and an initial investment of RM20 million, laying a solid foundation that adhered to stringent Good Manufacturing Practice (GMP) standards. Over 30 years, the company has transformed into an industry giant with 1,300 employees and a cumulative Direct Domestic Investment of RM450 million.
In its early days, AIN MEDICARE faced significant challenges, including limited financial capacity and scepticism from medical professionals toward products from a new homegrown company. Convincing banking institutions to provide financial assistance proved equally difficult, given the company’s status as an emerging industry player.
Operating from a single block that housed the office, laboratory, production area, and raw materials storage, AIN MEDICARE had to rent a nearby warehouse to store finished goods. Despite these hurdles, the company’s resilience and commitment to quality fuelled its exponential growth. Today, AIN MEDICARE operates from six (6) state-of-the-art buildings, a testament to its ability to adapt, expand, and meet ever-increasing demand while remaining a trusted name in the pharmaceutical industry.
A certified current Good Manufacturing Practice (cGMP) company since 1994, AIN MEDICARE was also selected as a facility to be audited for certification by the National Pharmaceutical Regulatory Agency (NPRA) and admission of Malaysia as a member of Pharmaceutical Inspection Co-operation Scheme (PIC/S). The company is also certified for Medical Device – Quality Management Systems (EN ISO 13485: 2016) issued by TUV SUD, Quality Management System (ISO 9001:2015) issued by Det Norske Veritas, Laboratory Management System (MS ISO/IEC17025) issued by Department of Standards Malaysia, and Environment Management System (ISO 14001:2015).
Patience and perseverance have successfully steered the company towards numerous remarkable achievements. AIN MEDICARE products have gained international acceptance and are used by both private and public health providers in Malaysia and abroad. AIN MEDICARE is significantly expanding its export network across diverse countries, including countries in the Caribbean, South America, Middle East and Southeast Asia. The company was also recently awarded the Fastest Growing Export Revenue (2019-2023) under the category of less than RM50 million by the Malaysian Organisation of Pharmaceutical Industries (MOPI) at their Pharma Industry Awards 2024 held in September 2024.

Having carved a niche in Malaysia and the region, Ain Medicare has taken a step further in promoting halal pharmaceutical products, as part of its added value to the customers. On 21st April 2014, the company successfully became the first company to be certified halal for Intravenous Solution products by the Department of Islamic Development Malaysia (JAKIM). Since then, more products have successfully been certified in the pipeline. Additionally, AIN MEDICARE was recently honoured with the Halal Pharmaceutical Excellence Award at the 2023 World Halal Excellence Awards, organised by the Halal Development Corporation (HDC) in Melaka.
The most valuable asset of any company is the people – the human capital. The 1,300 personnel are the core backbone of AIN MEDICARE and they take pride in training them consistently to improve their knowledge and skills. AIN MEDICARE also nurtures future talents through industrial internship and protégé programmes. It is an investment to unlock their untapped talent. Those who excel and are competent will be given support and recognition for career enhancement.

AIN MEDICARE’s success is shared with other local entrepreneurs where they provide support to the small and medium enterprises (SMEs) through their Vendor Development Programme. This is a cumulative effort in boosting the nation’s economy while providing a platform for the small businesses to grow. 104 local SME businesses have been developed involving logistics, primary and secondary packaging, component and engineering-based services, a testament of their commitment in nation building.

MIDA has assumed a key role in AIN MEDICARE’s success by providing grants for modernisation, helping them meet international standards, and expand their products and markets. With support from MITI, MIDA and other Government agencies, AIN MEDICARE has overcome obstacles and achieved growth, offering high-quality products comparable to imported drugs. They are optimistic about future growth and expansion.