This site
is mobile
responsive
The production of ventilator components and respiratory sub-systems in Malaysia has assumed a crucial role in managing the medical devices supply chain during the COVID-19 outbreak. Ventilation is an air exchange between the lungs and air, in moving air in and out of the lungs. The process’s most important effect is removing carbon dioxide (CO2) from the body while not increasing blood oxygen content. Ventilators, also known as respirators, breathing machine or mechanical ventilation are medical devices that are used to supply oxygen to patients when they cannot breathe independently.
Ventilators are divided into two categories; invasive/ mechanical ventilation and non-invasive ventilation. Non-invasive ventilation is used in patients with milder symptoms, in the form of facemasks, nasal masks or mouthpiece without the need for intubation to allow air or oxygen mixture to be pushed into the lungs.
Invasive ventilation is used in patients with severe symptoms where damage to the lungs has occurred, causing oxygen levels to drop, making it harder for patient to breathe. This procedure may also be used during surgery and recovery post-surgery.
To alleviate the patient, a ventilator will be used to push air, with increased oxygen levels, into the lungs and to remove the carbon dioxide from the body via either an endotracheal tube or tracheostomy tube.
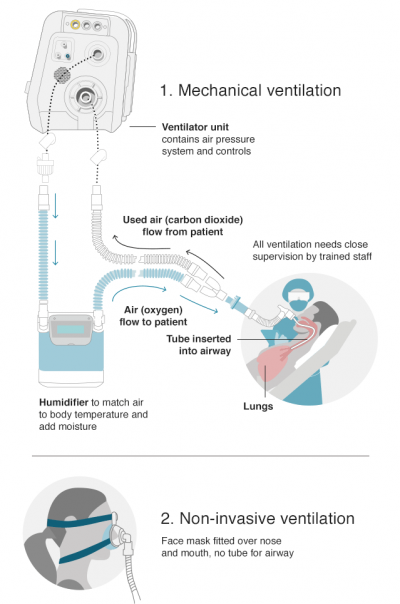
Source: BBC News, Coronavirus: What are ventilators and why are they important? 16 April 2020.
The global market for ventilators is expected to increase from USD1.05 billion in 2019 to USD3.02 billion by 2027 with a compound annual growth rate (CAGR) of 13.96 per cent. (Source: GlobeNewswire).
The ventilator market is currently witnessing a massive global demand due to high increase of diseases that attacks the lungs, such as SARS, Mers-CoV and the latest COVID-19. As at 15 March 2021, 120,417,029 COVID-19 cases had been reported with 2,665,246 deaths worldwide (Source: https://www.worldometers.info/ coronavirus/)
According to the World Health Organisation (WHO), some 80 per cent of people affected with COVID-19 recover without needing hospital treatment. However, one person in six becomes seriously ill, with the virus causing damage to the lungs and causing the body’s oxygen levels to drop, making it harder to breathe.
In the U.S., the Society of Critical Care Medicine estimates that by adding together full-featured and basic hospital ventilators, Strategic National Stockpile (SNS) ventilators, and hospital based anesthesia machines, there are only around 200,000 ventilators to treat Covid-19 patients in the US (Source: The Society of Critical Care Medicine; United States Resource Availability for COVID-19, 12 May 2020)- Publication. As at 15 March 2021, the US reported 30,081,657 cases with 7,365,186 active cases.
In the third wave of the Malaysian epidemic, the Malaysian Government has increased the supply of ventilators from 1,239 to 1,583 ventilators. Fortunately, as at 15 March 2021, only 4.5 per cent of the ventilators were being used as only 71 patients were reported to be on ventilator support.

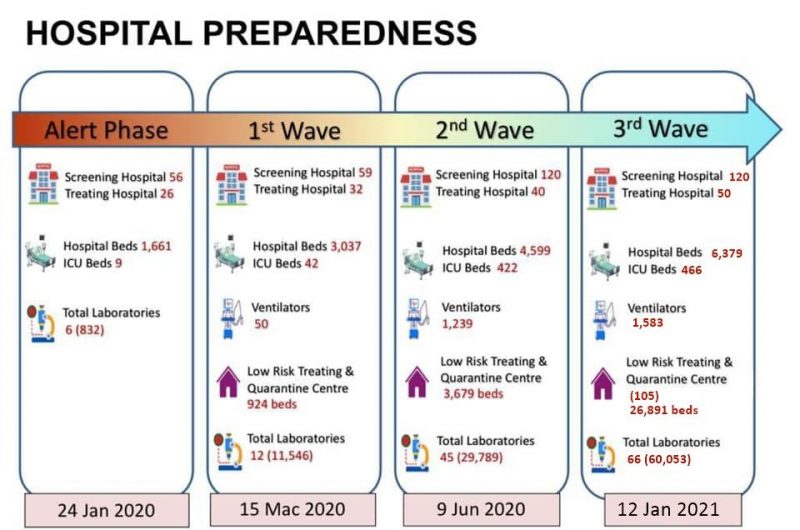
CPRC, hospital services, MOH
Source: CodeBlue: 30,000 Active COVID Cases Filling 91 per cent Beds, 13 January 2021.
Key operating players in the global ventilator market include Medtronic (Ireland), Becton, Dickinson and Company (USA), Teleflex Incorporated (USA), ResMed (USA), Koninklijke Philips N.V. (the Netherlands), Hamilton Medical (Switzerland), Allied Healthcare Products Inc. (USA) and General Electric Company (USA).
During the early phases of COVID- 19 in 2020, temporary trade restrictions by many countries on critical medical and pharmaceutical supplies had created a global supply chain disruption as manufacturers halted exports to meet the demand in their home market.
Malaysia needs to prepare to face similar pandemics in the near future. A mapping of the ventilator supply chain will allow Malaysia to leverage its strength in the electronic manufacturing services (EMS) sub-systems and components to meet the increase in demand for ventilators. It is utmost important that potential supplies are of ISO 13845 approved requirements or other medical device qualification requirements.
The shortage of ventilators and other medical supplies during the pandemic have also encouraged non traditional healthcare companies to take up the challenge to meet the international demands of healthcare products. In the US, Ford partnered with 3M and GE Healthcare to speed up respirators and ventilators’ production.
In Malaysia, K-One Technology Bhd (KOT) became the only company licensed to manufacture and distribute National Aeronautics and Space Administration (NASA) Jet Propulsion Laboratory’s (JPL) Ventilator Intervention Technology Accessible Locally (VITAL) ventilators. KOT has been granted a non-exclusive license agreement (NELA) to manufacture and distribute VITAL ventilators worldwide. VITAL has been approved for use in the US by the Food and Drug Administration (FDA) under the Emergency Use Authorisation (EUA) tailored for COVID-19 patients.
Product innovations for variants of basic ventilators are also being pursued to replace conventional ventilators. Saora Industries has produced Malaysia’s first-of-a-kind semiventilator, Ethovent, an automated bagging machine using traditional Ambu Bags, to assist patients with breathing difficulties.
It replaces the need for using a bag valve mask or “Ambu bag” with an innovated hand-held manual resuscitator. This project had also been earmarked as one of the pilot projects under the National Technology and Innovation Sandbox (NTIS), announced during the Economic Recovery Plan (PENJANA) in June 2020.
The COVID-19 pandemic has caught all off guard in the need and urgent supply of emergency tools and protocols, such as lifesaving ventilators. There is an urgent call to escalate ventilators’ production by manufacturers in Malaysia to meet domestic needs and international demands. The supply chain management for ventilators and alternative product innovation, from manufacturing, procurement, storage, distribution and delivery to the coronavirus frontline has to be established to meet unexpected circumstances.

Source: MIDA e-Newsletter February 2021